In a collaboration between the University of Bonn, the FAU Erlangen and Durham University, aequorin-based sensor lines of potato and Arabidopsis was used to analyse and compare Ca2+ transients in response to different abiotic and biotic stimuli using soil grown plants. The analysis revealed differences in the kinetics and amplitude of Ca2+ transients between both species, implying species-specific sensitivity for different stress conditions.
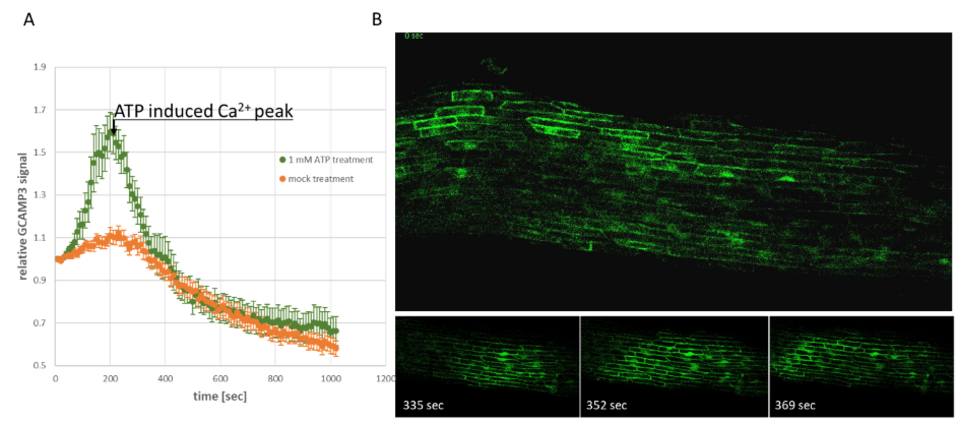
To further elucidate the Ca2+ responses with a better resolution in regards to specific tissues and even specific cells, a potato line carrying the fluorescence-based genetically encoded Ca2+ indicator GCaMP3 (StGCaMP3) was generated. The indicator localizes in the cytosol and nucleus and due to the power of regions-of-interest provide by high-resolution microscopes, changes in the Ca2+ levels of both compartments can be analysed separately. Changes in GCaMP3 fluorescence in response to external application of ATP (Figure 1A) and stress-induced Ca2+ waves going through the stolon tissue can be observed using a fluorescence microscope (Figure 1B).
This new sensor line will become part of our ongoing studies on early signaling events in Désirée under drought/heat and flooding stress to ultimately generate a detailed timeline and comprehensive event map of early stress responses. Moreover, they will be used to address questions regarding Ca2+ signaling in stolon development and cell specific stress response, e.g. in stomata.